
Through its management of materials, it has an ability to handle a variety of analytical techniques such as IR, Raman, NIR, FIR, UV-Vis, thermal analysis, DSC and TGA, XRPD, EELS, NMR, MS, chromatography, and others, enabling the companies to choose favorite chemicals without any hurdle.
#CHEMDOODLE DELOCALIZED BENZENE SOFTWARE#
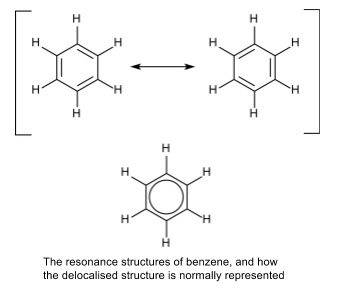
“Benz4” By Selfmade by cacycle, Leyo – Own work (CC BY-SA 3.ACD/ChemSketch is one of the reliable software that lets you draw chemical structures such as organics, polymers, organometallics, and Markush structures. “Delocalized Electron.” Wikipedia, Wikimedia Foundation, 13 July 2020, Available here. “Localized and Delocalized Lone Pairs and Bonds.” Chemistry Steps, 22 Aug. Moreover, delocalized electrons are associated with particular atoms in a compound while the delocalized electrons are associated with all the atoms in the molecule. The key difference between localized and delocalized electrons is that localized electrons are located between atoms, whereas delocalized electrons are located above and below the atoms. The terms localized and delocalized electrons are discussed under general chemistry. Summary – Localized and Delocalized electron The following table summarizes the differences between localized and delocalized electrons. Besides, localized electrons are graphically indicated by straight lines, while delocalized electrons are graphically indicated by circles. Moreover, another significant difference between localized and delocalized electrons is that the localized electrons are associated with particular atoms in a compound while the delocalized electrons are associated with all the atoms in the molecule. In other words, localized electrons are confined to a particular region between two atoms while delocalized electrons are spread over several atoms. A localized atom is an electron that belongs to a particular atom while a delocalized electron is an electron not associated with any single atom or a single covalent bond. We use terms localized and delocalized electrons under the branch of general chemistry, regarding the chemical structure of compounds. What is the Difference Between Localized and Delocalized Electrons? Moreover, quantum physics use the term delocalized electrons to refer to molecular orbital electrons that have extended over several atoms.
#CHEMDOODLE DELOCALIZED BENZENE FREE#
In solid-state physics, delocalized electrons are the free electrons that facilitate electrical conduction. For example, in organic chemistry, delocalized electrons are in the resonance structures of conjugated systems in aromatic compounds. However, the term delocalized electron has different meanings in different fields. This term refers to electrons that are not associated with a single atom or a covalent bond. What are Delocalized Electrons?ĭelocalized electrons are the nonbonding electrons in chemical compounds. Localized electrons are shared between atoms forming covalent bonds, coordination bonds, etc. These localized electrons belong to two particular atoms, in contrast to delocalized electrons, which are common to all the atoms in the molecule. Therefore, localized electrons occur in covalent compounds having covalent chemical bonds. Sigma bonds are the bonds formed by the axial overlapping of half-filled atomic orbitals of atoms. These electrons are located between atoms where sigma bonds can be found. Localized electrons are the bonding electrons in chemical compounds. Side by Side Comparison – Localized vs Delocalized Electrons in Tabular Form Localized electrons are the bonding electrons in molecules while delocalized electrons are nonbonding electrons that occur as electron clouds above and below the molecule. In general chemistry, localized electrons and delocalized electrons are terms that describe chemical structures of chemical compounds.
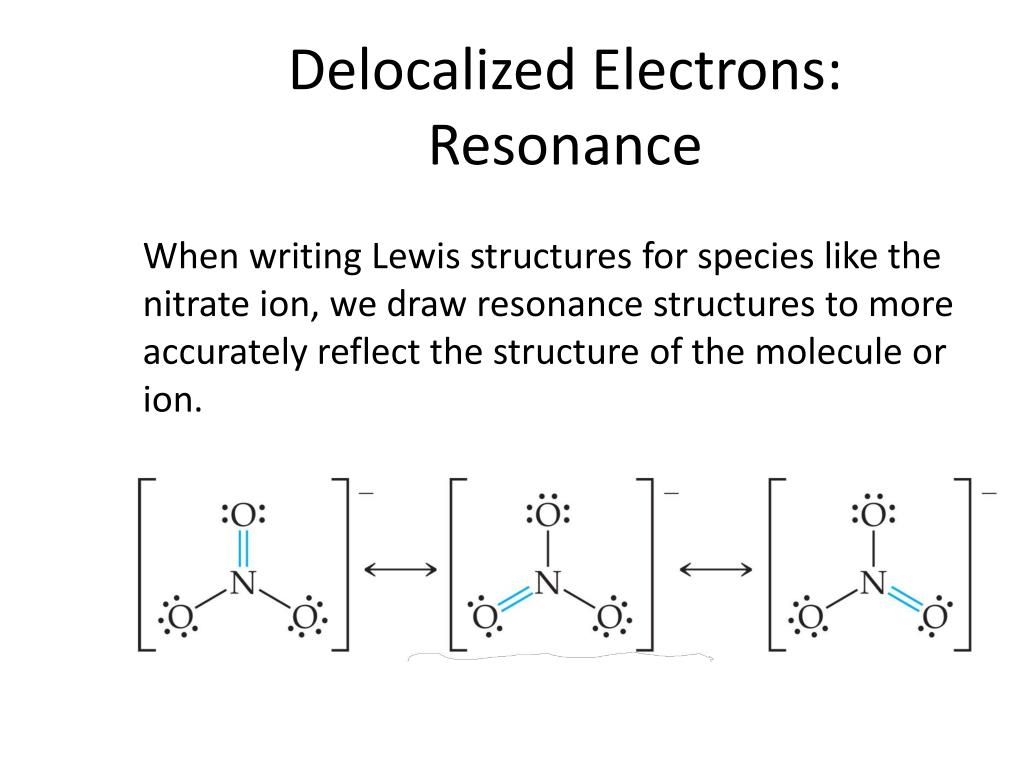
The key difference between localized and delocalized electrons is that localized electrons are located between atoms, whereas delocalized electrons are located above and below atoms.


 0 kommentar(er)
0 kommentar(er)
